THE ENDOCANNABINOID SYSTEM
The Endocannabinoid System (ECS)
First, allow us to preface our introduction and description by stating we are not interested in, nor are we advocating the administration of, the well-known psychotropic compound Δ9-tetrahydrocannabinol (Δ9-THC), in concentrations sufficient to produce the ‘high’ or psychotropic effects which are normally associated with marijuana. We are not advocating the administration of marijuana, by any route, in any form, to any animal for the purposes of producing medical benefit or to induce any psychotropic effect.
Rather, we are advocating the administration and continued research into the compounds called phytocannabinoids, terpenes and flavonoids for the purposes of primary, alternative and integrative therapy in companion animals.
Over 20 years ago, we became interested in the well-established and ongoing research from Europe, Canada and Israel concerning the more than 545 identified chemical compounds in Cannabis sativa L., or hemp. The ratioed combination of phytocannabinoids, terpenes and flavonoids has been and continues to be scientifically researched concerning their ability to support and modulate numerous physiologic and pathophysiologic processes. This section of the website describes the interaction between these compounds and the structure and function of the endocannabinoid system (ECS).
Early research was focused on high dosage form single compounds, derived from Cannabis species. However, over the last decade, the research paradigm emphasis has shifted to multiple compounds in low to low-moderate quantities. This shift away from single to multiple ratioed compound formulations and the pharmacodynamic alteration, has and continues to yield the most significant results over a diverse range of pathophysiologic conditions. It is this approach, which has been our focus since inception and remains one of the fundamental principles in our research and development.
The ECS is ubiquitous in vertebrates and has currently been identified in 60% of invertebrates. It consists of four identified receptor subtypes, signaling pathways, endogenous ligands (endocannabinoids – eCB), endocannabinoid membrane transporters (EMT) and the biosynthetic and catabolic pathways for said ligands.
Endocannabinoids (eCB)
There are six known eCBs, of which the most abundant and extensively researched are 2-arachidonoylglycerol (2-AG) and N-arachidonoylethanolamine (anandamide). In simplistic terms, these are endogenous forms of CBD and THC respectively, as they closely approximate the receptor binding and response observed with exogenous phytocannabinoid administration. The other lesser understood compounds are 2-arachidonoyl-glyceryl-ether (noladin ether), O-arachidonoyl-ethanolamine (virodhamine), N-arachidonoyl dopamine (NADA) and possibly oleamide.
Endogenous cannabinoids are classified as neurotransmitters, neuromodulators and immunomodulators. Additionally, these compounds, whether endogenous or exogenous, are extremely lipophilic, and their main actions are accomplished via paracrine and autocrine signaling. Moreover, the ECS exerts control over several neurotransmitters. This direct or indirect regulation includes: glutamate, gamma-aminobutyric acid (GABA), glycine, noradrenaline, serotonin, dopamine, acetylcholine and neuropeptides. The ECS imparts its own discrete physiologic effects, but also directly or indirectly moderates numerous other receptor systems. In its most simplistic description, the ECS is a homeostatic receptor system listening for inflammatory mediators, stress catecholamines and increased sympathetic tone.
Finally, the ECS is classified as a retrograde signaling system. For example, they act as retrograde messengers in GABAergic and glutamatergic synapses and as modulators of postsynaptic transmissions, concerning their interaction with other neurotransmitters. Simply explained, endocannabinoids (eCBs) are synthesized postsynaptically and interact with cannabinoid receptors (CBRs) located on the presynaptic button.
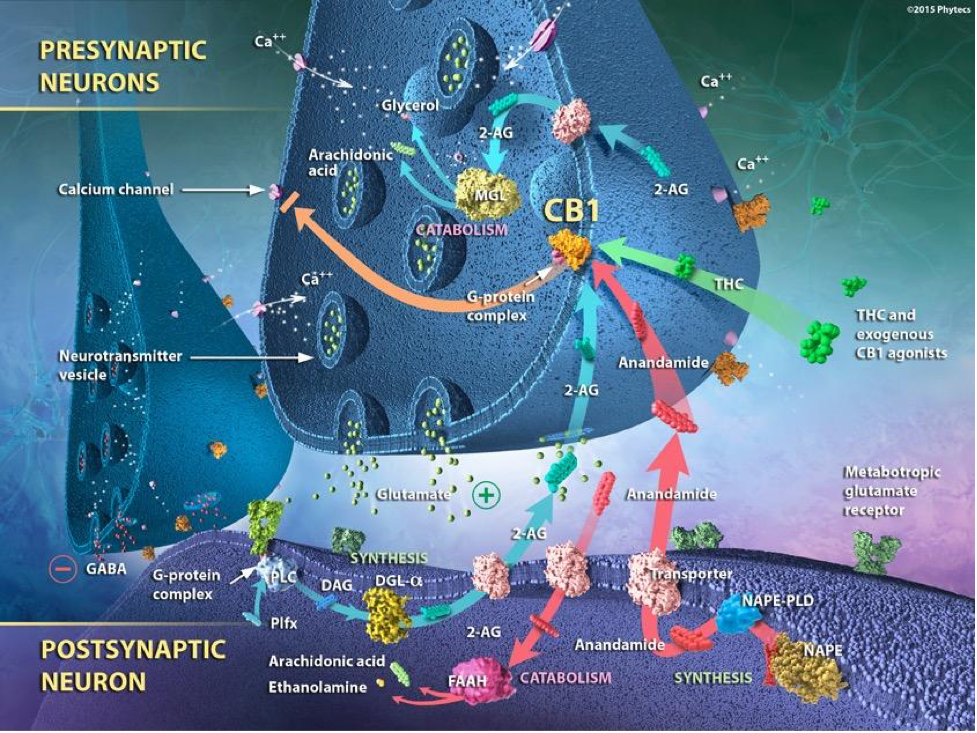
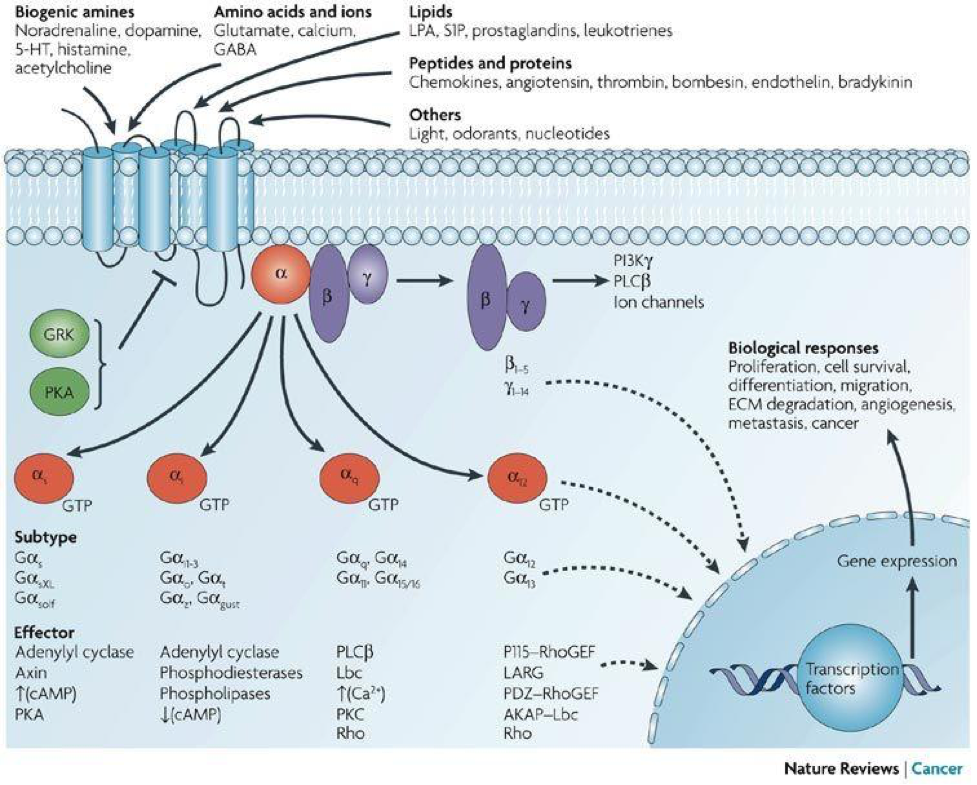
G-Protein Coupled Receptors (GPCR)
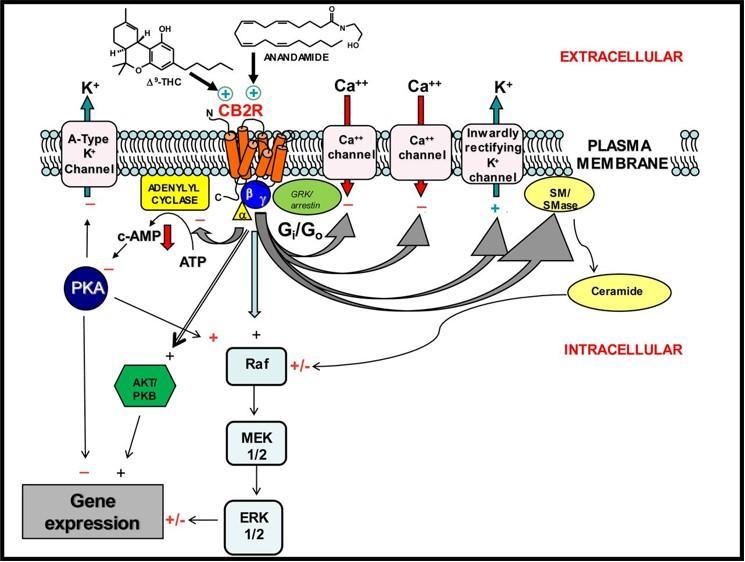
The other components of the ECS are the 7-transmembrane-domain G-protein coupled cannabinoid receptors (GPCR), of which CB1R and CB2R appear to be the most abundant. GPCRs act as guanine nucleotide exchange factors for the α subunit of the G protein, whereby an activated receptor promotes the exchange of bound guanine diphosphate (GDP) for guanosine-5′-triphosphate (GTP) on the α subunit, which is the rate-limiting step in G protein activation. The binding of GTP changes the conformation of ‘switch’ regions within the α subunit, which allows the bound trimeric G protein (inactive) to be released from the receptor, and to dissociate into active α subunit (GTP-bound) and βγ dimer. The α subunit and the βγ dimer go on to activate distinct downstream effectors, such as adenylyl cyclase, phosphodiesterases, phospholipase C, proto-oncogene tyrosine-protein kinase (Src), and ion channels. The ion channels include, calcium (Ca2+), potassium (K+) and sodium (Na+).
These effectors in turn regulate the intracellular concentrations of secondary messengers, such as cyclic adenosine monophosphate (cAMP), cyclic guanosine monophosphate (cGMP), diacylglycerol (DAG), inositol trisphosphate IP3, arachidonic acid (ARA), sodium (Na+), potassium (K+) or calcium cations (Ca2+), which ultimately lead to a physiological response, usually via the downstream regulation of gene transcription. The cycle is completed by the hydrolysis of a subunit-bound guanosine-5′-triphosphate (GTP) to guanine diphosphate (GDP), resulting in the re-association of the α and βγ subunits and their binding to the receptor, which terminates the signal.
Distribution of Endocannabinoid Receptors
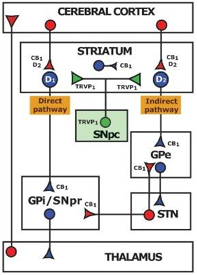
The distribution of CB1R is primarily in the CNS, PNS, and ANS. CB1R is one of the most abundant and widely expressed G protein-coupled receptor in the mammalian brain. CB1R is expressed in the olfactory bulb, neocortex, entorhinal cortex, piriform cortex, hippocampus (extensive concentration), amygdala (extensive concentration), several parts of basal ganglia, thalamic and hypothalamic nuclei, cerebellar cortex, neocortex, medial prefrontal cortex (vMPFC), cornu ammonis (CA), nucleus accumbens (NAcc), dentate gyrus, medulla oblongata, brainstem nuclei and grey matter of the spinal cord.
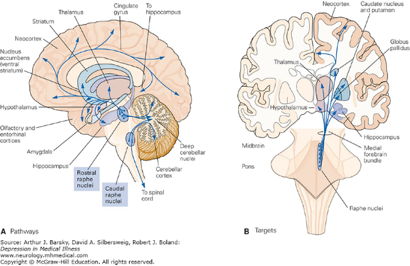
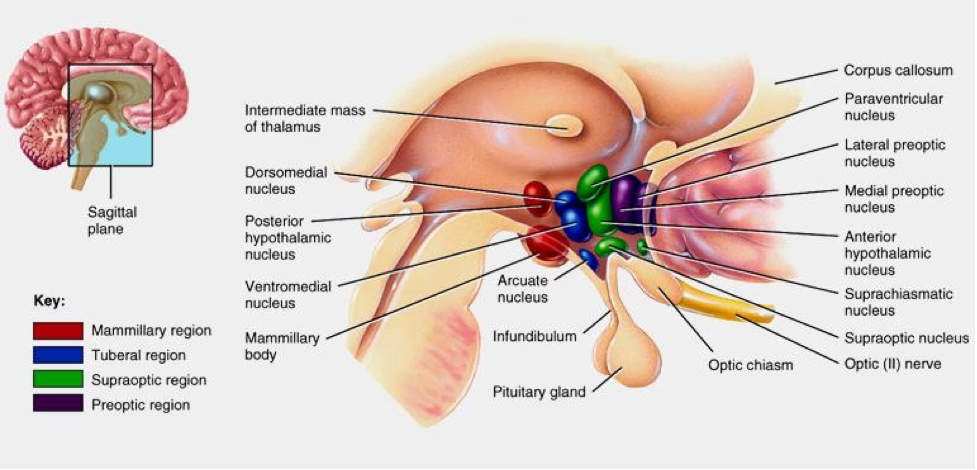
In subcortical regions, CB1R is present at relatively high levels in the septal region (lateral and medial septum, and vertical and horizontal nuclei of the diagonal band). Dense expression is found in the fibers of the Globus pallidus and substantia nigra surrounding immunonegative neurons. Some expression is observed in the medial and lateral preoptic hypothalamic nucleus, magnocellular preoptic nucleus, and paraventricular nucleus (PVN). In the caudal hypothalamus, CB1R expression is demonstrated in the premammillary nucleus. In the lateral hypothalamus, CB1R is present in scattered cells. In the thalamus, CB1R is present in the lateral habenula, reticular thalamic nucleus, and zona incerta. There are some CB1R present in tyrosine hydroxylase-expressing neurons in the ventral tegmental area (VTA) and in dopaminergic terminals in the striatum. CB1R is also found in the anterior and intermediate lobes of the pituitary. Astrocytes, satellite cells of the dorsal root ganglia and myelinating Schwann cells express moderate to dense concentrations of CB1R, as well.
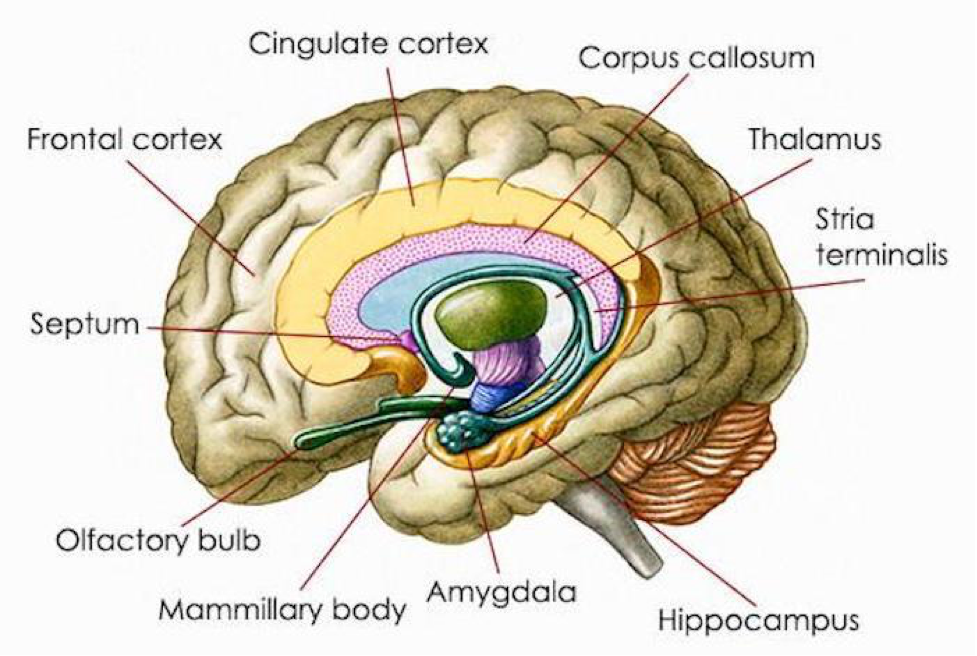
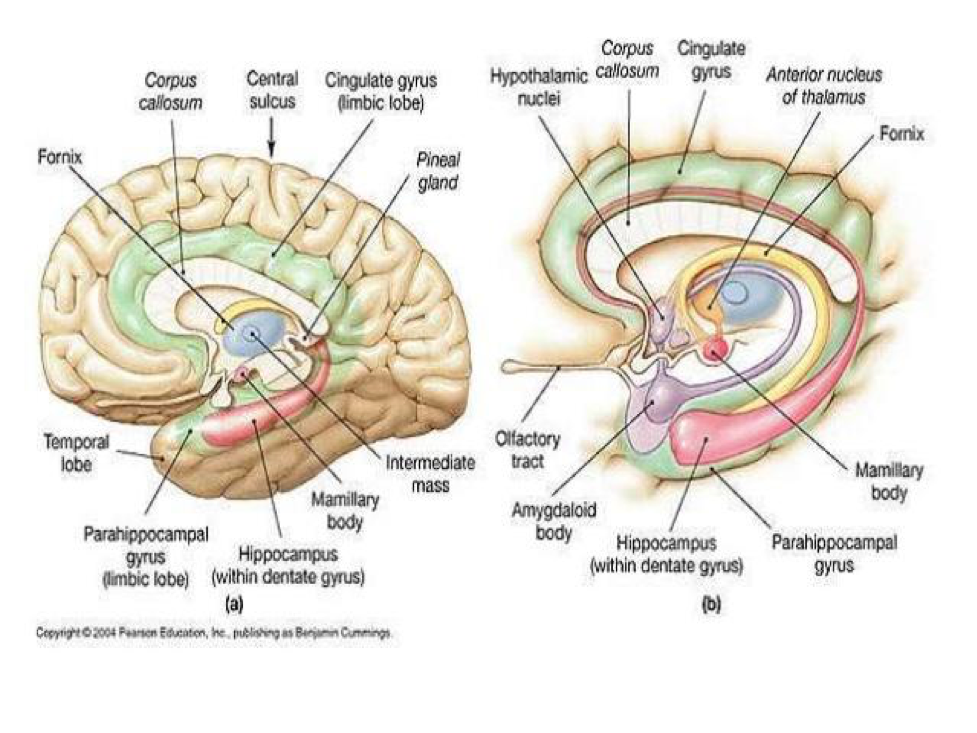
Finally, thyroid, adrenal, male and female reproductive system, hepatic, adipocytes, pulmonary, renal, myocardium, vascular, gastrointestinal neuronal tissues all contain CB1R.
The distribution of CB2R is primarily the immune system, spleen, tonsils, thymus, gastrointestinal tract, osteocytes, monocytes, macrophages, microglia, B-cells and T-cells. However, the CB2R is also found in varying concentrations in the following areas: cerebral cortex, hippocampal pyramidal cells, Globus pallidus, cerebellar Purkinje cells, cerebellar granule cells, cerebellar nuclei, vestibular nuclei, dorsal motor nucleus of the Vagus, nucleus ambiguus, spinal trigeminal nucleus, substantia nigra and spinal sensory neurons.
As it relates to the immune system and the inflammatory cascade, CB2R exhibit both cellular and humoral modulation. The following cellular hierarchy represents immune cells which either produce endocannabinoids or present cannabinoid receptors on their cell surface or both: macrophages, monocytes, natural killer cells (NKC), lymphocytes, mast cells, CD8+ and CD4+. Other cells which interrelate with the ECS are: leukocytes, B cells, dendritic cells, platelets, microglia and Kupffer cells. Humoral ECS interaction is observed with the following interleukins: IL1-α/β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-14, IFNγ and TNFα. The ECS is also involved in the protein kinase C (PKC) pathway and as currently understood, to a lesser degree in protein kinase A and B pathways.
The concluding two receptors were recently discovered and the extent of their distribution and effects is currently being researched. CB4R appears to be predominately located in endothelium and its function is not fully understood but may be involved in vascular tone and endothelial cytokine interaction. CB6R and its distribution is unclear, and it appears to act in an anti-inflammatory capacity, though in a different manner than that of CB1R and CB2R.
The ECS and Cross-Reactivity with Other Receptor Systems
Orphan Receptors (GPCR)
An ever-increasing segment of the research community hypothesizes several of the orphan receptors are mislabeled cannabinoid receptors. The following receptors listed, either interact directly with eCB, exogenous cannabinoids or their biosynthetic and catabolic enzymes or the significant secondary and tertiary cannabinoid metabolites, of which there are currently 115 identified for Δ9-THC and 108 for CBD. The orphan receptors which demonstrate ECS interaction are:
- GPR3, found in oocytes
- GPR6, up-regulates cyclic adenosine monophosphate (cAMP) levels and promotes neurite outgrowth
- GPR12, found in the limbic system and promotes brain growth
- GPR18, roles in microglial migration/inflammation and peripheral vasodilation – expressed in the rostral ventromedial medulla (RVM)
- GPR55, osteoclast function and neuroimmunological regulation
- GPR119, expressed in the pancreas and gastrointestinal tract and may have a role in glucose homeostasis.
There are several others, but this research is in the nascent stages.
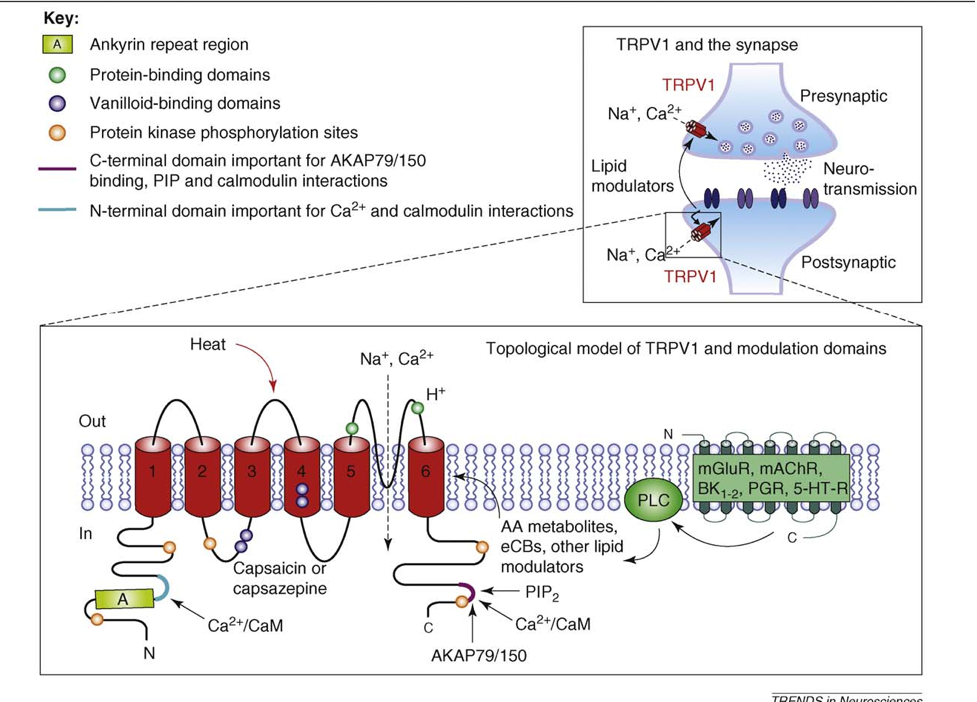
Transient Receptor Potential Vanilloid (TRPV) and Transient Receptor Potential Melastatin (TRPM)
Additionally, there is interaction between the ECS and vanilloid receptors: TRPV1, TRPV2, TRPV3, TRPV4. The vanilloid receptors (TRPV) are ligand-gated, non-selective cation channels expressed predominantly by sensory neurons. Research concerning TRPV1 is increasing and current results indicate significant interaction with a normally functioning inflammatory cascade. There is also ECS interaction with the melastatin non-selective cation channel receptor TRPM8.
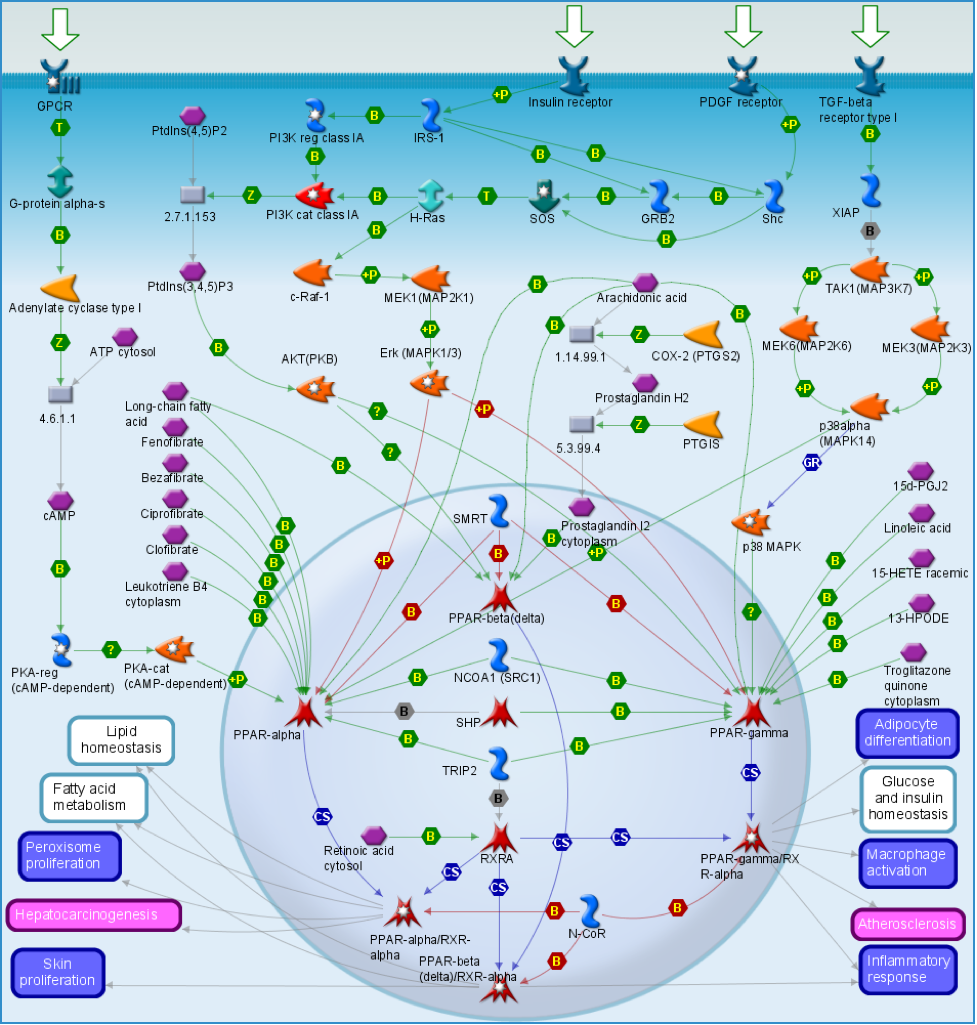
Peroxisome Proliferator-Activated Receptors (PPAR)
ECS interaction is observed between peroxisome proliferator-activated receptors (PPAR). PPAR receptors are ligand-activated transcription factors of nuclear hormone receptor superfamily comprised of the following three subtypes: PPARα, PPARγ, and PPARβ or δ. Activation of PPAR-α reduces triglyceride level and is involved in regulation of energy homeostasis. Activation of PPAR-γ causes insulin sensitization, enhances glucose metabolism and may inhibit inflammatory cytokines, whereas activation of PPAR-β/δ enhances fatty acid metabolism. Thus, the PPAR family of nuclear receptors play a major regulatory role in energy homeostasis, metabolic function, immune regulation and inflammatory cytokine production and regulation.
Gamma-Aminobutyric Acid (GABA) and Glutamate
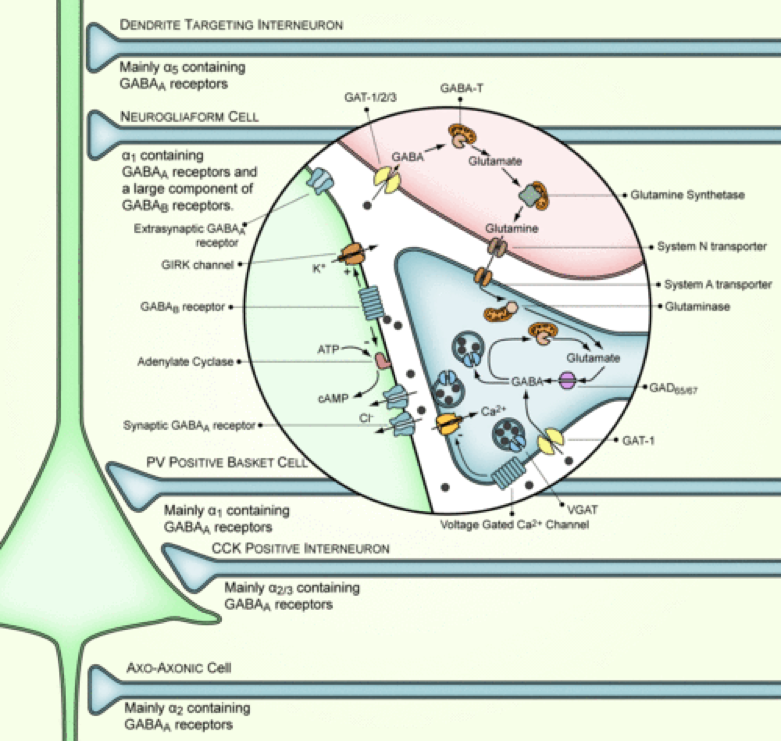
The neurotransmitter group including gamma-aminobutyric acid (GABA), GABAergic interneurons, glutamate, glutamatergic neurons and cholecystokinin (CCK), is interrelated with the endocannabinoid system. The ECS inhibits hippocampal GABA release, GABAergic transmission and network oscillations. Additionally, the ECS has also been demonstrated to increase glutamatergic transmission in the hippocampus. Furthermore, it modulates glutamate and decreases GABAergic synaptic transmission in the amygdala. CB1R is found overwhelmingly on the nerve terminals of a distinct group of GABAergic interneurons, which besides GABA, also contain the neuropeptide cholecystokinin (CCK). Similarly, CB1R is found in the cerebellum and striatum in high concentration on the terminals of the excitatory glutamatergic fiber systems.
Endocannabinoids, depending on the brain region, can regulate the release of GABA, cholecystokinin or glutamate with precision. In part, this regulation has demonstrated ability to modulate the different phases of memory processes, namely acquisition, consolidation, retrieval, reconsolidation and extinction. Moreover, the regulation plays a role in plasticity and neuronal depolarization control.

Opioid Receptors (OPRM1 and OPRD1)
There exists extensively detailed research revealing a significant correlation and interaction between the ECS and OPRM1 (mu) and OPRD1 (delta) receptors. This is confirmed and observed in a bidirectional upregulation between CB1R and the OPR1 receptors in the periaqueductal gray (PAG), rostral ventromedial medulla (RVM), locus coeruleus (LC), dorsal horn, peripheral unmyelinated C-fibers and some distribution in myelinated A fibers. It is important to understand that the ECS is interwoven into all phases of the pain cascade: transduction, transmission, perception and modulation. The ECS modulates C-fibers and ‘wind up’ pain by decreasing sensitivity to substance P, reducing sprouting and altering N-methyl-D-aspartate (NMDA) and glutamate concentrations.

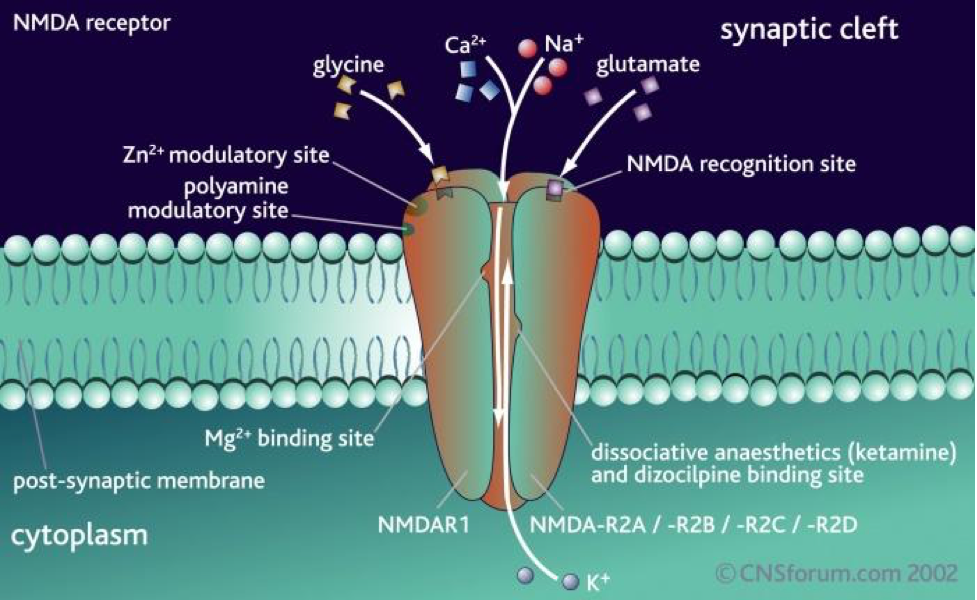
N-methyl-D-aspartate Receptors (NMDAR)
Cross reactivity has been observed between N-methyl-D-aspartate Receptors (NMDAR) and the ECS, which is involved in the production and binding of N-methyl-D-aspartate (NMDA). The distribution of NMDAR is throughout most structures in the brain, as well as in the cervical spinal cord, dorsal root and vestibular ganglia, and in pineal and pituitary glands. Moderate to dense concentration is observed in the olfactory bulb, neocortex, striatum, some thalamic and hypothalamic nuclei, the colliculi, and many reticular, sensory, and motor neurons of the brainstem and spinal cord. The densest concentration appears in the pyramidal and hilar neurons of the CA3 region of the hippocampus, Purkinje cells of the cerebellum, supraoptic and magnocellular paraventricular neurons of the hypothalamus, inferior olive, red nucleus, lateral reticular nucleus, peripheral dorsal cochlear nucleus, and motor nuclei of the lower brainstem and spinal cord.
These receptors are glutamate-gated cation channels with high calcium permeability. They are critical for the development of the central nervous system (CNS), generation of rhythms for breathing and locomotion, and the processes underlying learning, memory, and neuroplasticity.
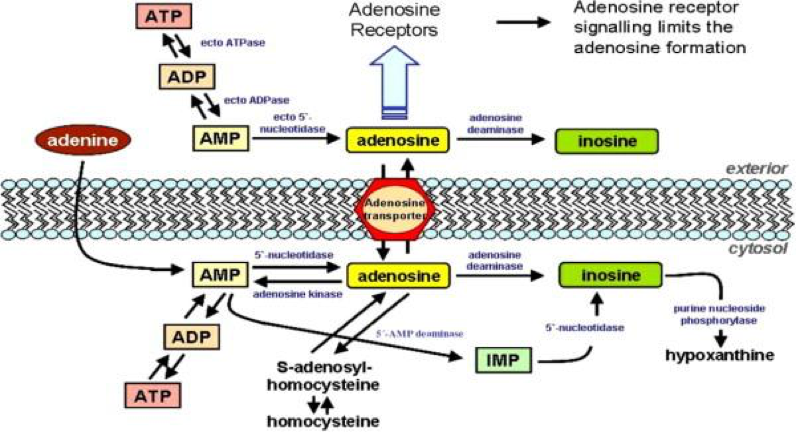
Adenosine Receptors (AR)
Another receptor group which exhibits ECS interaction, is the adenosine receptor A3. It is found in high concentrations in the testes and mast cells, in moderated concentrations in the cerebellum and hippocampus and in low concentrations in the thyroid, most of brain, adrenal gland, spleen, liver, kidney, and heart. It imparts cardioprotective function during cardiac ischemia. A3 helps inhibit neutrophil degranulation in neutrophil-mediated tissue injury, has both neuroprotective and neurodegenerative effects, and may also mediate both cell proliferation and cell death.
Concerning cell death, the ECS modulates several components of apoptosis, including several caspases. It is an inhibitor of B-cell lymphoma 2, a protein-coding gene, which causes increased mitochondrial membrane permeability and functions in a feedback loop with caspases. Caspases are endoproteases that are proteolytic peptidases which break peptide bonds of nonterminal amino acids, i.e. within a molecule. The specific caspases controlled by the ECS are the initiators, 8 and 9, the executioners 3, 6 and 7 and common 2 and 10.
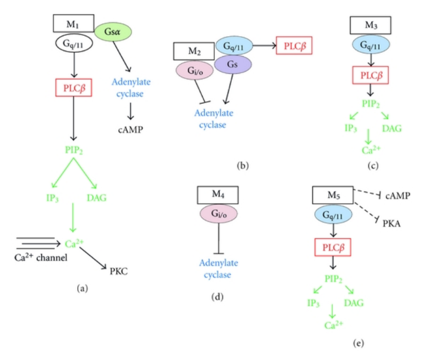
Muscarinic Acetylcholine Receptors (mAChR)
There is interaction between muscarinic M1R and M4R and the ECS. M1R is found in the hippocampal and cortical regions of the brain as well as in the parasympathetic ganglia. It is involved in the initiation of neuronal excitation, learning and memory, and regulation of the force and rate of heart contractions. M4R is abundant in neostriatum, the cortex and hippocampus. Its function is thought to mediate an inhibitory effect on striatal dopamine-mediated locomotor activity and appears to have smooth muscle effects as well.
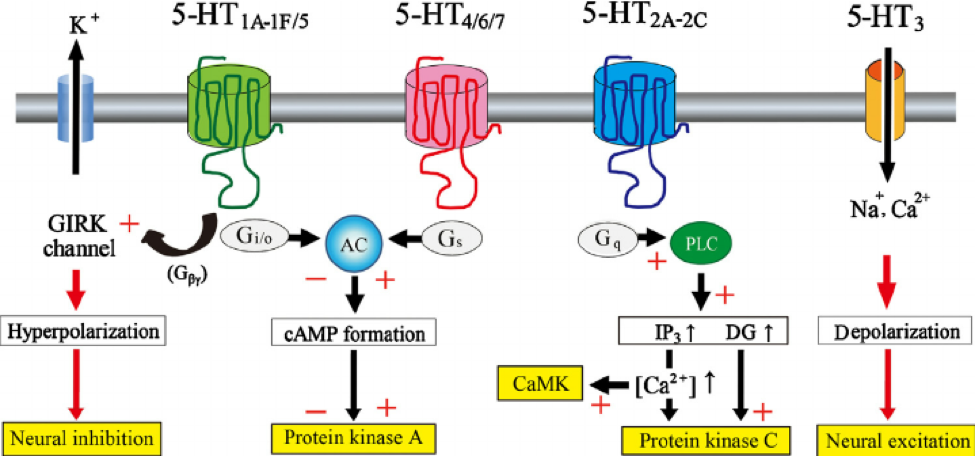
Serotonin Receptors (5HT)
The 5HT1AR, 5HT2AR and 5HT3AR serotonin receptors exhibit substantial interaction with the ECS. 5HT1AR inhibit adenylyl cyclase and open K+ channels. It is widely distributed throughout the CNS and are present in both pre and postsynaptic sites. Presynaptically, 5HT1ARs are exclusively located on cell bodies and dendrites of 5HT (serotonin) neurons in the dorsal and median raphe nuclei, and function as 5HT1AR auto receptors which tightly regulate 5HT (serotonin) neuronal activity.
Postsynaptically, the highest level of 5HT1AR is found in the limbic system, specifically in the entorhinal cortices, cingulate and lateral septum, with particularly high expression in the hippocampus. At the cellular level, the postsynaptic 5HT1AR is expressed in cortical pyramidal neurons as well as pyramidal, GABAergic and granular cells of the hippocampus. In the hippocampus, the 5HT1AR is located on somata and dendrites of pyramidal and granular neurons, as well as on the dendritic spines of pyramidal neurons. Neuronal hyperpolarization occurs with activation of 5HT1AR and leads to an effect mediated by pertussis-toxin-sensitive Gαi/o proteins. Gαi/o proteins are negatively coupled with the signaling pathway of adenylyl cyclase and thereby decrease the cyclic adenosine monophosphate (cAMP) formation.
5HT2AR stimulate phosphoinositide-specific phospholipase C and close potassium (K+) channels. Their location is in post-synaptic membranes of 5HT (serotonin) target cells and are widely distributed in many brain areas, including limbic regions such as the hypothalamus, amygdala, nucleus accumbens (NAcc), striatum, hypothalamus, and prefrontal cortex (PFC). Additionally, it is found in the claustrum, cerebral cortex and olfactory tubercle. This receptor has been shown to play a role in appetite, temperature and blood pressure regulation, neuroendocrine function and behavior.
5HT3AR are ligand-gated ion channels (LGIC) and therefore differ from all other 5HT (serotonin) receptors whose actions are mediated via G proteins. They are primarily expressed on GABAergic interneurons in neocortex and limbic structures, derived from the caudal ganglionic eminence. However, they are also found in the following areas: hippocampus, entorhinal cortex, amygdala, nucleus accumbens (NAcc), solitary tract nerve, trigeminal nerve, motor nucleus of the dorsal vagal nerve, area postrema, dorsal horn of the spinal cord.
Dopamine Receptors (D1 and D2)
The ECS correlates with the dopamine receptors, DR1 and DR2. D1R is widely expressed in the brain, with the highest levels found in the caudate-putamen, nucleus accumbens (NAcc), substantia nigra, pars reticulata, and olfactory bulb. They are associated with learning, memory, locomotor activity and reward mechanisms. D2R is highly expressed in the caudate, putamen (basal ganglia), nucleus accumbens (NAcc), ventral tegmental area and the substantia nigra and in lower concentrations in the septal region, amygdala, hippocampus, thalamus, pituitary gland, cerebellum and cerebral cortex. Specifically, in the cerebellum the highest concentrations are in lobules IX and X. D2R can be located both presynaptically, where it regulates the release of dopamine and other neurotransmitters, and postsynaptically, where it can exert a variety of functions, ranging from inhibition of long-term depression at midbrain excitatory synapses, to inhibition of calcium channels, to control of pacemaker activity and resting potential through activation of G protein-coupled inwardly-rectifying potassium channels (GIRK).
Nuclear Factor – Kappa B (NF-kB)
Nuclear factor-Kappa B (NF -KB ), represents a family of inducible transcription factors that regulate a large array of genes involved in numerous processes related to immune and inflammatory responses. NF-KB regulates numerous aspects of innate and adaptive immune functions and serves as a pivotal mediator of inflammatory response. NF-KB induces the expression of various pro-inflammatory genes, including those encoding cytokines and chemokines. Additionally, this transcription factor participates in inflammasome regulation.
Moreover, NF-KB plays a critical role in regulating the proliferation, survival, activation, and differentiation of innate immune cells and inflammatory T cells. The ECS modulates and controls this transcriptive factor via numerous processes, which are responsible for inflammation, cellular metabolism, mitochondrial activity, immune modulation, and redox mechanisms. Communication is not one-sided. Innate immune cells express cannabinoid receptors and produce endogenous cannabinoids. Hence, innate immune cells regulate endocannabinoid homeostasis, in part.
ECS modulation of NF-kB, demonstrates several facets of the interactions observed between the ECS and other receptor systems, as well as the specific interaction and communication with Toll-like receptors (TLR) and the homeostatic function of the ECS via primary eCANNS (endogenous and exogenous cannabinoids) and their constituent metabolites. To provide some sense of interaction extent, the ECS interacts with the following immune representative cells: natural killer cells, polymorphonuclear cells, leukocytes, monocytes, CD4+, CD8 +, lymphocytes, dendritic cells, and macrophages.
Now, expand to the processes involved in the production, maturation, distribution, survival, interaction, and degradation of these cells. What do those processes inhibit, modulate, or stimulate? How many heterodimers and or homodimers form and interact with what number of receptor systems? We are learning and research and clinical experience are filling the blanks. It is complex; it takes money, support, and time. The ECS is intricate and it is this complexity that affords the possibility to modulate the ECS in a personalized way, for specific pathophysiologies, via designed eCANNS formulations and administration regimes.
More specifically, the NF-kB interaction demonstrates how integral the ECS is concerning inflammatory process control and modulation and immune function and response. The extent of this interaction is below.
ECS interaction with NF-kB
THC/CBD and eCANNS, through CB1, CB2, and TVRP1, have a direct inhibitory action on TNF a, NF-kB, and IFN-y. This action is accomplished via an initial interaction with T helper type 1 cells (Th1). Furthermore, modulation of macrophages, neutrophils, and Th cells, decreases the expression of IL-113, IL-6, IL 10, TNF-a and TGF-b for macrophages, leading to an alteration of either pro or anti-apoptotic caspase production. NF-kB can function as both in a single cell type. Neutrophils are blocked from producing IL-6, TNF-a, ROS, and NOS which results in the modulation of caspases. Th1 cells are blocked from the production of TNF-a, IFN-y, and IL-2. Th2 cells are blocked from the production of IL-4, IL-5, and IL-10. Th17 cells are blocked from the production of IL-17. Dendritic cell maturation and interaction with Th are modulated.
The interaction reiterates the importance of both CBD and THC in achieving a cascading homeostatic interaction. THC is a potent and talented immune modulator, as well as possessing several other equally significant single and combined effects. In the ECS/NF-kB interaction, each compound interacts with its own modifying qualities and overlapping potentiating effects, demonstrating the entourage effect.
This is the ECS DNA repeating, the inherent trait within the coding and wiring of the ECS, and the reaction to the multitude of compounds found within the Cannabis genus. Research has observed this multi-pathway interaction and potentiation in each of the pathophysiologies for which the ECS is scientifically investigated, analgesia, behavior, antiepileptic, oncologic, inflammation, etc. I feel this trend will persist.
The following is a brief compilation, detailing the potential of THC and providing a comparison concerning the effects of THC and CBD, focusing only on immune system functions.
- THC
- Decreases nociception, via CB2
- Reduces neuropathic nociception, via peroxisome proliferator activated receptors (PPARs).
- Modulates via transient receptor potential channel (TRPA1) activation.
- TRPA1 is part of the superfam1ly of transient receptor potential channel receptors (TRP). These are ion gated, plasma membrane channels. Categorized into 2 groups, consisting of a total of ten (10) channels.
- They are involved in the perception of a wide range of chemical and physical stimuli, including temperature and osmolarity changes, light, sensation, taste, and pheromones, and upon activation initiate cellular responses.
- Modulates IFN-y biosynthesis.
- Modulates IL-2 biosynthesis.
- Modulates serotonin biochemical pathways.
- Decreases T cell expression and proliferation.
- CBD
- Decreases the intensity of neuropathic sensation via PPARs.
- Increases protection for nerve fiber via antioxidant effects, against redox mechanisms.
- Modulates antigen expression on T cells during major challenges to the immune system.
- Increases analgesic effects through FAAH inhibition and thus increase anandamide (AEA) expression.
- Increases anti-proliferative effects on T-cell line.
- Modulates responses through TRPA1.
- Alters pro-inflammatory expression via PPARs.
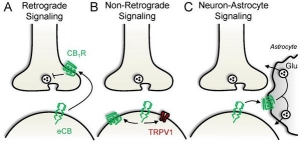
The final point on this transcriptive factor interaction demonstrates the three types of ECS signaling and how they can be utilized to aid in the modulation of the immune system, achieving multiple health goals by harnessing the numerous receptor systems under ECS homeostatic control. This is possible within a multitude of full-spectrum formula frameworks if they are focused on supporting and potentiating the inherent entourage coding in both the ECS and the Cannabis genus. In doing so, ECS support can be formulated for multiple organ systems and pathophysiologies. The following signaling definitions are another modifiable aspect of the ECS.
- Retrograde signaling – Pre-synaptic interaction of eCANNS, including THC, with both CB1 and CB2.
- Non-retrograde signaling – Post-synaptic produced and exogenous cannabinoids interact with post-synaptic CB1, CB2, and TRVP1.
- Neuron – Astrocyte signaling – Post-synaptic endocannabinoids stimulate astrocyte CB1, thereby triggering gliotransmission. This is seen in neuronal tissue glucose and glutamate modulation.
The neuron-astrocyte signaling pathways are distributed as named, which makes these networks crucial in neurodegenerative, traumatic, behavioral, anxiety, and brain originating pathophysiologies. I feel this signaling pathway is critical and under-studied. It is an important receptor path that remains receptive in multiple neurologic based conditions when others may be hyporesponsive. ECS integration and distribution in the brain is extensive and thus these pathways are equally disseminated.
The ECS Biosynthesis and Catabolic Compounds
The final component of the ECS are the biosynthetic and catabolic compounds responsible for production, regulation and degradation of endogenous ligands, and degradation of exogenous phytocannabinoids. These also work to degrade exogenous phytocannabinoids as well. Compounds included in these processes are:
- fatty acid amide hydrolase (FAAH)
- monoacylglycerol lipase (MAGL)
- diacylglycerol lipase (DAGL α and β)
- phospholipase C (PLC)
- phosphatase
- phosphatidylinositol
- N-arachidonoyl phosphatidylethanolamine (NAPE)
- N-acyl phosphatidylethanolamine phospholipase D (NAPE-PLD)
- N-acyl phosphatidylethanolamine-hydrolyzing phospholipase C (NAPE-PLC)
- 1,2-diacylglycerol (DAG)
- N-acetyltransferase (NAT)
- cyclooxygenase-2 (COX2)
- lipoxygenase (LOX)
- 2-arachidonoylglycerol hydrolase (ABDH6)
- α,β-hydrolase-12 (ABDH12)
